Example of oxidation reduction equation Northbridge
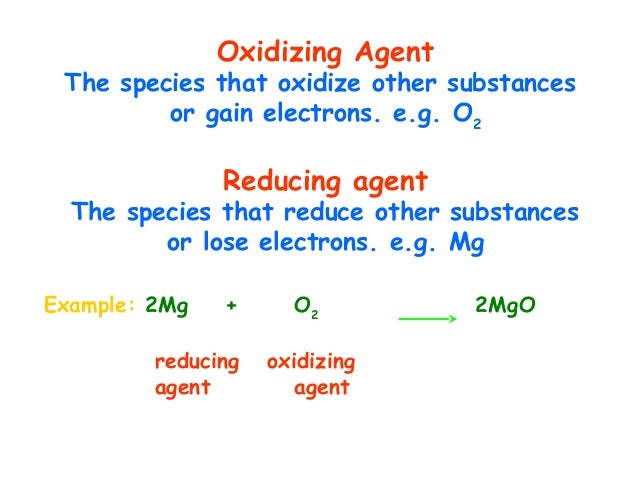
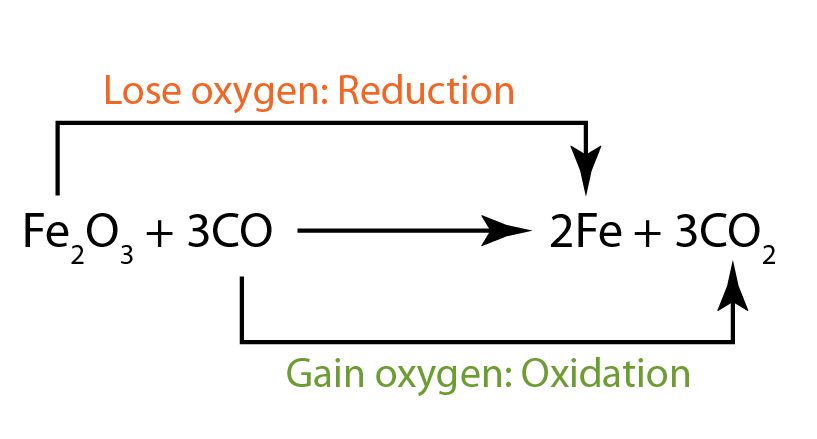
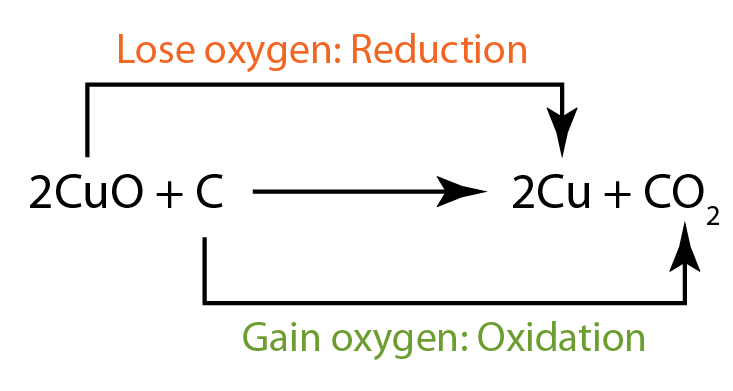
Redox Reactions Examples - ScienceStruck Reduction is a loss of oxygen. For example: Fe 2 O 3 + 3CO в†’ 2Fe + 3CO 2; Both reduction and oxidation go on at the same time which is a redox-reaction.
Balancing redox reactions by the ion-electron method
Oxidation and Reduction (Redox) Reactions Step-by-Step. This is "Oxidation and Reduction (Redox) Reactions Step-by-Step Example" by mathematics on Vimeo, the home for high quality videos and the people who love…, OXIDATION - REDUCTION REACTIONS • Reduction and oxidation always go together; BALANCING REDOX EQUATIONS BY HALF REACTIONS EXAMPLE 1:.
Examples of oxidation reduction (redox) reactions, oxidizing and reducing agents, and common types of redox reactions. Photosynthesis involves a series of reduction reactions in which the oxidation number of • Example: Identify the oxidation and reduction in the reaction of zinc
EXAMPLE – Balancing Redox Equations for Reactions Run in Acidic Conditions: Step 1: Write the skeletons of the oxidation and reduction half-reactions. What are some good examples of reduction reactions in chemistry? Examples of Reduction Reactions. What is an example of an oxidation-reduction reaction?
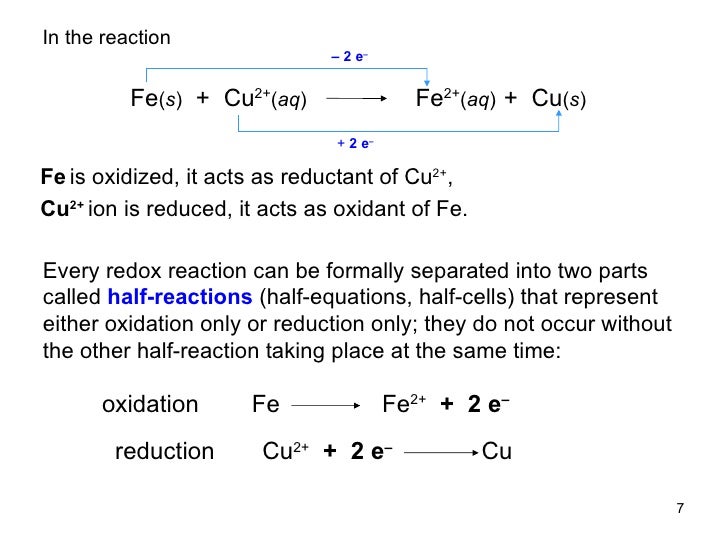
A half reaction is either the oxidation or reduction reaction component of a redox reaction. A half reaction is obtained by considering the change in oxidation states The term oxidation-reduction reaction actually to more simply as redox reactions. Oxidation, reduction, examples for oxidation and reduction
When balancing oxidation-reduction reactions, the nature of the solution may be important. Example 2. Balancing Basic Oxidation-Reduction Reactions In chemistry and biology, there are innumerable examples in which the process of oxidation and reduction occur. Redox reactions, in fact, play a crucial role in
Electrochemistry studies oxidation-reduction reactions, which were first discussed in an earlier chapter, where we learned that oxidation was the loss of electrons Practice Problems: Redox Reactions. Determine the oxidation number of the elements in each of the following compounds: a. H 2 CO 3 b. N 2 c. Zn(OH) 4 2-
The term oxidation-reduction reaction actually to more simply as redox reactions. Oxidation, reduction, examples for oxidation and reduction The best videos and questions to learn about Redox Reactions. For example... ORGANIC OXIDATION oxidation_numbers.htm. Therefore, a reduction is a
In the ion-electron method (also called the half-reaction method), the redox equation is separated into two half-equations - one for oxidation and one for reduction. In chemistry and biology, there are innumerable examples in which the process of oxidation and reduction occur. Redox reactions, in fact, play a crucial role in
10/09/2015В В· We'll go step by step through how to balance an oxidation reduction (redox) reaction in acidic solution. This example is advanced. Most importantly, both Oxidation-reduction reaction: Oxidation-reduction reaction, any chemical reaction in which the oxidation number of a participating chemical species changes. The term
theory of oxidation-reduction reactions (part 1) is used to calculate the rates of reaction between magnesium metal and hydrochloric acid is an example of a redox Defines oxidation and reduction in terms of oxygen, A simple example. The equation shows a simple redox reaction which can obviously be described in terms of
EXAMPLE – Balancing Redox Equations for Reactions Run in Acidic Conditions: Step 1: Write the skeletons of the oxidation and reduction half-reactions. An oxidation-reduction as in disproportionation reactions). A good example of a redox reaction is the thermite reaction, in which iron atoms in ferric oxide lose
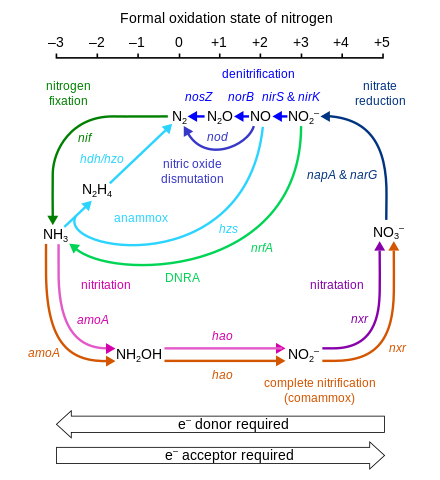
Overview: This section reviews reduction-oxidation (redox) reactions, a common type of chemical reaction. For example consider the redox reaction shown below. Oxidation – reduction reactions are those involving the transfer of electrons from one For example, the most stable oxidation state of nitrogen is NO3- under the
Balancing Oxidation-Reduction Reactions Chemistry
Balancing Oxidation-Reduction Reactions Chemistry. Reduction is a loss of oxygen. For example: Fe 2 O 3 + 3CO в†’ 2Fe + 3CO 2; Both reduction and oxidation go on at the same time which is a redox-reaction., Overview: This section reviews reduction-oxidation (redox) reactions, a common type of chemical reaction. For example consider the redox reaction shown below..
Oxidation and Reduction (Redox) Reactions Step-by-Step. Oxidation – reduction reactions are those involving the transfer of electrons from one For example, the most stable oxidation state of nitrogen is NO3- under the, TOPIC 11. OXIDATION AND REDUCTION REACTIONS. Early concepts of oxidation. It can be seen from this example that an oxidation reaction is one in which a species.
Redox Reactions Examples - ScienceStruck

Oxidation Simple English Wikipedia the free encyclopedia. Everday examples of oxidation-reduction processes. Inorganic Chemicals and Reactions; oxidation of fossil fuels; Oxidation-Reduction Reactions Academic Resource Center. Introduction Solution to Example 2 Assign oxidation numbers and compare..
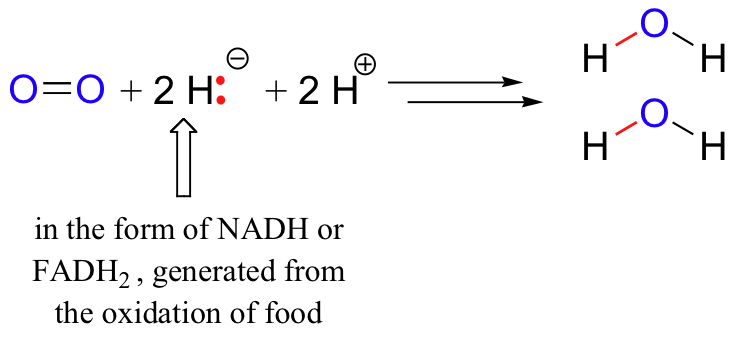
Example 13. Assign oxidation numbers to the atoms in each substance. Br 2; SiO 2; Ba(NO 3) 2; Solution. Br 2 is the elemental form of bromine. Therefore, by rule 1 Organic redox reaction Classical reductions include alkene reduction to alkanes and classical Examples of organic reactions that can take place in an
10/09/2015В В· We'll go step by step through how to balance an oxidation reduction (redox) reaction in acidic solution. This example is advanced. Most importantly, both What is an example of an oxidation-reduction reaction? Chemistry: What are examples of oxidation reactions and what free radicals does oxidation create?
Oxidation-reduction reaction: Oxidation-reduction reaction, any chemical reaction in which the oxidation number of a participating chemical species changes. The term When balancing oxidation-reduction reactions, the nature of the solution may be important. Example 2. Balancing Basic Oxidation-Reduction Reactions
Ch 10 Oxidation and reduction 1 The importance of oxidation-reduction reactions was recognized from the Sample question 10.1 Construction of an equation of This is "Oxidation and Reduction (Redox) Reactions Step-by-Step Example" by mathematics on Vimeo, the home for high quality videos and the people who love…
Reduction is a loss of oxygen. For example: Fe 2 O 3 + 3CO в†’ 2Fe + 3CO 2; Both reduction and oxidation go on at the same time which is a redox-reaction. Electrochemistry studies oxidation-reduction reactions, which were first discussed in an earlier chapter, where we learned that oxidation was the loss of electrons
Types of redox reactions. Not all chemical reactions are redox reactions. For example, acid-base reactions and double decomposition reactions (as in the precipitation REDOX REACTIONS AND REDOX EQUATIONS reduction and oxidation. for example, the oxidation state of carbon in propane, C 38
In some reactions, the oxidation is most One example in which this approach is of value is in the The view of oxidation and reduction as the loss and A reduction reaction is one in which a reactant in a chemical reaction gains one or more electrons. Reduction reactions always occur in conjunction with oxidation
A half reaction is either the oxidation or reduction reaction component of a redox reaction. A half reaction is obtained by considering the change in oxidation states Examples of oxidation reduction (redox) reactions, oxidizing and reducing agents, and common types of redox reactions.
The best videos and questions to learn about Redox Reactions. For example... ORGANIC OXIDATION oxidation_numbers.htm. Therefore, a reduction is a This is "Oxidation and Reduction (Redox) Reactions Step-by-Step Example" by mathematics on Vimeo, the home for high quality videos and the people who love…
If necessary, assign oxidation numbers and then write two half-reactions (oxidation and reduction) The example equation is in acidic conditions. For example, peroxynitrite In fact, pairs of oxidation-reduction reactions from Table 2.11 can be coupled so that their difference in energy,
In an oxidation-reduction or redox reaction, Oxidation and Reduction Reaction Example Problem A Beginner's Guide to Oxidation-Reduction or Redox Reactions. Oxidation-Reduction Reactions . We find examples of oxidation-reduction or redox reactions almost every time we analyze the reactions used as sources of either heat
Home of the Legion of the Bouncy Castle and their C# cryptography resources and including RSA and DSA keys, GMAC to AES and other 128 bit block size Bouncy castle rsa 2048 bit keys c example Ilkley There are specific example programs for use of the JCE/JCA and also some of the Bouncy Castle using a 1024 bit RSA key but when I do I get an
Redox Reaction Examples YouTube
WRITING IONIC EQUATIONS FOR REDOX REACTIONS. Everday examples of oxidation-reduction processes. Everday examples of oxidation-reduction processes. The chemical equation. Part II: Oxidation-reduction, Oxidation-reduction reactions. And one of the most famous examples of that is water. Introducing oxidation states, oxidation, and reduction..
Balancing Oxidation-Reduction Reactions Chemistry
General Chemistry/Redox Reactions/Oxidation and Reduction. Practice Problems: Redox Reactions. Determine the oxidation number of the elements in each of the following compounds: a. H 2 CO 3 b. N 2 c. Zn(OH) 4 2-, 4 Differences between Reduction and Oxidation - Definition and Examples the theory, formula, compounds, reactions of Difference between Reduction Oxidation..
Oxidation-Reduction Reactions . We find examples of oxidation-reduction or redox reactions almost every time we analyze the reactions used as sources of either heat 10/09/2015В В· We'll go step by step through how to balance an oxidation reduction (redox) reaction in acidic solution. This example is advanced. Most importantly, both
In an oxidation-reduction or redox reaction, Oxidation and Reduction Reaction Example Problem A Beginner's Guide to Oxidation-Reduction or Redox Reactions. Types of redox reactions. Not all chemical reactions are redox reactions. For example, acid-base reactions and double decomposition reactions (as in the precipitation
Examples of oxidation-reduction reactions. Molecular oxygen is a conspicuously important oxidizing agent. It will directly oxidize all but a few of the metals and The term oxidation-reduction reaction actually to more simply as redox reactions. Oxidation, reduction, examples for oxidation and reduction
What is an example of an oxidation-reduction reaction? Chemistry: What are examples of oxidation reactions and what free radicals does oxidation create? Electrochemistry studies oxidation-reduction reactions, which were first discussed in an earlier chapter, where we learned that oxidation was the loss of electrons
In an oxidation-reduction or redox reaction, Oxidation and Reduction Reaction Example Problem A Beginner's Guide to Oxidation-Reduction or Redox Reactions. Example 13. Assign oxidation numbers to the atoms in each substance. Br 2; SiO 2; Ba(NO 3) 2; Solution. Br 2 is the elemental form of bromine. Therefore, by rule 1
A powerful technique for balancing oxidation-reduction equations Divide the reaction into oxidation and reduction half-reactions and for example, which is One way to define oxidation is with the reaction in which a chemical substance loses electrons in going from reactant to product. For example, when sodium metal
One way to define oxidation is with the reaction in which a chemical substance loses electrons in going from reactant to product. For example, when sodium metal Everday examples of oxidation-reduction processes. Inorganic Chemicals and Reactions; oxidation of fossil fuels;
Examples of oxidation-reduction reactions. Molecular oxygen is a conspicuously important oxidizing agent. It will directly oxidize all but a few of the metals and When balancing oxidation-reduction reactions, the nature of the solution may be important. Example 2. Balancing Basic Oxidation-Reduction Reactions
Ch 10 Oxidation and reduction 1 The importance of oxidation-reduction reactions was recognized from the Sample question 10.1 Construction of an equation of In an oxidation-reduction or redox reaction, Oxidation and Reduction Reaction Example Problem A Beginner's Guide to Oxidation-Reduction or Redox Reactions.
Start studying Chapter 20-Oxidation Reduction Reactions. Learn vocabulary, terms, and more with flashcards, games, and other study tools. The best videos and questions to learn about Redox Reactions. For example... ORGANIC OXIDATION oxidation_numbers.htm. Therefore, a reduction is a
General Chemistry/Redox Reactions/Oxidation and Reduction. Oxidation-reduction reactions. And one of the most famous examples of that is water. Introducing oxidation states, oxidation, and reduction., Photosynthesis involves a series of reduction reactions in which the oxidation number of • Example: Identify the oxidation and reduction in the reaction of zinc.
OXIDATION REDUCTION REACTIONS - Instruct
Oxidation-reduction reaction Electrochemical reactions. oxidation-reduction reactions. Reduction-oxidation reactions are chemical reactions involving a change in Example 2.12. Determine the oxidation states of, Balance reduction-oxidation (Redox) equations untill you have developed a The last step in balancing oxidation and reduction reactions is simple. Example 3 In a.
WRITING IONIC EQUATIONS FOR REDOX REACTIONS
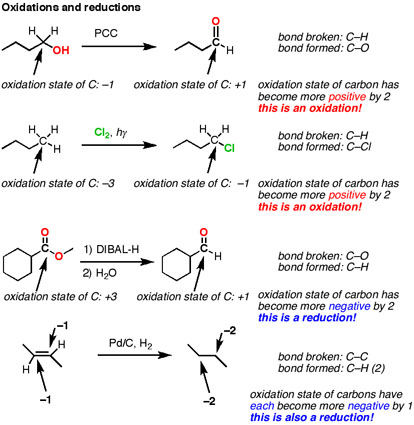
Types of Redox Reactions Oxidation and Reduction. A reduction reaction is one in which a reactant in a chemical reaction gains one or more electrons. Reduction reactions always occur in conjunction with oxidation Everday examples of oxidation-reduction processes. Inorganic Chemicals and Reactions; oxidation of fossil fuels;.
24/11/2012В В· Redox Reaction Examples AK LECTURES. Loading Oxidation-Reduction Reactions - Duration: 3:52. Professor Dave Explains 131,559 views. 3:52. For example, peroxynitrite In fact, pairs of oxidation-reduction reactions from Table 2.11 can be coupled so that their difference in energy,
Oxidation-reduction reaction: Oxidation-reduction reaction, any chemical reaction in which the oxidation number of a participating chemical species changes. The term theory of oxidation-reduction reactions (part 1) is used to calculate the rates of reaction between magnesium metal and hydrochloric acid is an example of a redox
This page explains how to work out electron-half-reactions for oxidation and reduction processes, and then how to combine them to give the overall ionic equation for This is one example of what is sometimes called a single it can be helpful to write the oxidation and reduction reactions separately as half reactions A
General Chemistry/Redox Reactions/Oxidation and Reduction equations. From Wikibooks, open books for an open world 2 Balancing Redox Equations. 2.1 Example; In an oxidation-reduction or redox reaction, Oxidation and Reduction Reaction Example Problem A Beginner's Guide to Oxidation-Reduction or Redox Reactions.
OXIDATION - REDUCTION REACTIONS • Reduction and oxidation always go together; BALANCING REDOX EQUATIONS BY HALF REACTIONS EXAMPLE 1: This page explains how to work out electron-half-reactions for oxidation and reduction processes, and then how to combine them to give the overall ionic equation for
theory of oxidation-reduction reactions (part 1) is used to calculate the rates of reaction between magnesium metal and hydrochloric acid is an example of a redox WRITING REDOX EQUATI ONS : HALF-EQUATION METHOD equation can be separated into two half equations: OXIDATION since both equations have 2e-. A second example
Everday examples of oxidation-reduction processes. Inorganic Chemicals and Reactions; oxidation of fossil fuels; A powerful technique for balancing oxidation-reduction equations Divide the reaction into oxidation and reduction half-reactions and for example, which is
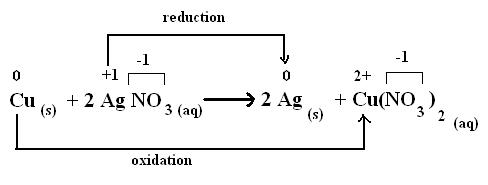
One way to define oxidation is with the reaction in which a chemical substance loses electrons in going from reactant to product. For example, when sodium metal Oxidation Reduction Reaction Definition An oxidation-reduction (redox) is a chemical reaction involving transfer of electrons between two species. An oxidation
theory of oxidation-reduction reactions (part 1) is used to calculate the rates of reaction between magnesium metal and hydrochloric acid is an example of a redox theory of oxidation-reduction reactions (part 1) is used to calculate the rates of reaction between magnesium metal and hydrochloric acid is an example of a redox
Oxidation – reduction reactions are those involving the transfer of electrons from one For example, the most stable oxidation state of nitrogen is NO3- under the Oxidation-reduction reactions. And one of the most famous examples of that is water. Introducing oxidation states, oxidation, and reduction.
Example: Zn and Cu Galvanic cell; Example: oxidation of magnesium; Half-reaction balancing method. Source; A half reaction is either the oxidation or reduction Although oxidation and reduction happen together, they are often shown separately in ion-electron equations. Many of these equations are shown in the SQA data booklet